Critical Temperature of Methanol
In the present work we applied scaled model to calculate surface tension vapor densities and the critical temperatures of four different models of methanol. And CRC Handbook of Chemistry and Physics 44th ed.
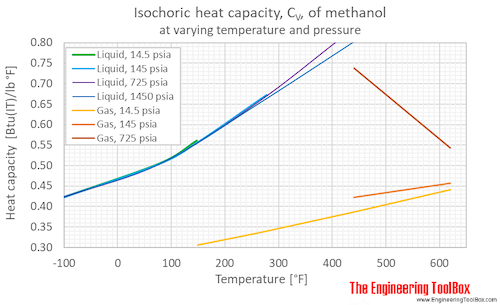
Methanol Specific Heat Vs Temperature And Pressure
The annotation d aCbC indicates density of solution at temperature a divided by density of pure water at temperature b known as specific gravity.

. Namely H1 J1 J2 and L1 models. What is the reduced temperature if the actual temperature is 200ºC. In biodiesel production processes using supercritical methanol the phase equilibria for methanol fatty acid methyl ester systems near the critical temperature of methanol T c 5126 K are required as the fundamental data.
In this paper we used scaled model by Hale to estimate vapor density surface tension and the critical temperature for the four different methanol models specifically H1 J1 J2 and L1. Belg 1904 18 18-55. The first system consists of n 1 interacting molecules and a.
In the present work we applied scaled model to calculate surface tension vapor densities and the critical temperatures of four different models of methanol. The scaled model is based on calculating the free energy difference between two systems. See Table 1 below Molecular weight.
When temperature b is 4 C density of water is. A flow type method was applied to measure the vapor-liquid equilibria for methanol fatty acid methyl ester binary. The first system consists of n 1 interacting molecules and a.
Enthalpy of phase transition. Critical-temperature and coexistence-curve measurements in thick films. The molecular parameters for methanol ethanol 1-propanol 1-butanol.
The scaled model is based on calculating the free energy of the system. This problem has been solved. The measurements were performed in the single-.
Cyclohexane--methanol Full Record. It used to be known as wood alcohol due to the fact that it was first produced by wood distillation. The isochoric heat capacity of pure methanol in the temperature range from 482 to 533K at near-critical densities between 27487 and 33159kgm3 has been measured by using a high-temperature and high-pressure nearly constant volume adiabatic calorimeter.
Physical Properties of Pure Methanol Molecular Weight 3204 g mol-1 Boiling Point Critical Temperature 5125K 760 mm Hg 1013 kPa 646C 239C. Entropy of phase transition. This system exhibits relatively simple Type I phase behavior and several groups have published.
The critical pressure of acetic acid is 5786 bar. The isochoric heat capacity of pure methanol in the temperature range from 482 to 533 K at near-critical densities between 27487 and 33159kg m3 has. Its main uses are in the petrochemical industry as a raw material for the production of Methanol ammonia acetylene.
Data obtained from Langes Handbook of Chemistry 10th ed. The critical temperature of this model is 515K which is very close to the. Mixtures of C02 and methanol were selected for the initial investigation of the solvatochromic behavior in supercritical fluid systemsThis combination is of interest as it combines the low critical temperature and pressure of carbon dioxide with a polar less volatile modifier.
The critical temperature of methanol is 5126K. Methanol CH 3 OH is a colorless polar liquid that is miscible with water. The scaled model is based on calculating the free energy difference between two systems.
Free energy calculations were performed by applying the Bennet acceptance ratio BAR using Monte-Carlo simulations at. 463F 1483F Critical Pressure 8084MPa Freezing Point-976C 785 atm -1437F Critical Density 02715 g cm-3 Reid Vapour Pressure 32 kPa. Δ vap H.
111 rows Properties of aqueous methanol solutions. Free energy calculations were perfo. I critical solution temperatures their application to the preparation of pure methyl ethyl and propyl alcohols and anhydrides II variations in the critical solution temperature in the normal alcoho Bull.
In this paper we used scaled model by Hale to estimate vapor density surface tension and the critical temperature for the four different methanol models specifically H1 J1 J2 and L1. The scaled model is based on calculating the free energy of the system. Namely H1 J1 J2 and L1 models.
What is the reduced pressure if the actual pressure is 10 bar.
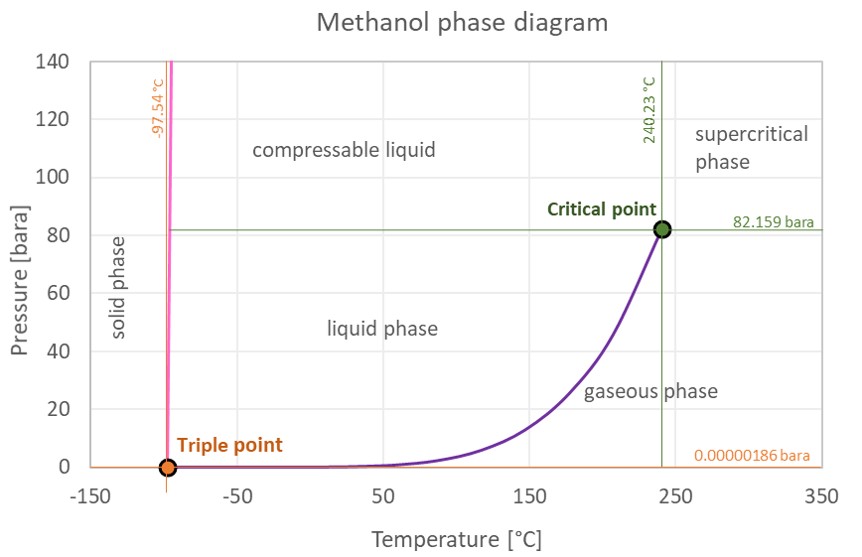
Methanol Thermophysical Properties

The Liquidus Temperature For Methanol Water Mixtures At High Pressure And Low Temperature With Application To Titan Dougherty 2018 Journal Of Geophysical Research Planets Wiley Online Library

Methanol Flammable Range As A Function Of Oxygen Percentage Download Scientific Diagram

Supercritical Solvents And Their Critical Temperature And Pressure Download Table
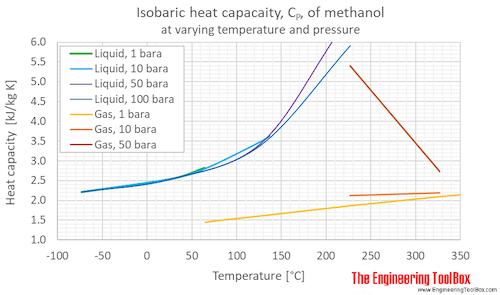
Methanol Specific Heat Vs Temperature And Pressure

0 Response to "Critical Temperature of Methanol"
Post a Comment